Columvi Combination Therapy Shows Statistically Significant Improvement in DLBCL Survival Rates
Roche recently announced the results of the STARGLO study at the European Hematology Association (EHA) 2024 Congress. The study found that Columvi® (glofitamab) in combination with gemcitabine and oxaliplatin (GemOx) reduces the risk of death by 41% in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). This is a significant development for the treatment of DLBCL, as historically, the standard second-line therapy for R/R DLBCL patients has been high-dose chemotherapy followed by stem-cell transplant, which is not always a viable option for everyone.
Background on DLBCL
DLBCL is the most common form of non-Hodgkin lymphoma (NHL), accounting for around one in three cases of NHL. While it is generally responsive to treatment in the frontline, up to 40% of people will relapse or have refractory disease. Salvage therapy options at this stage are limited, and survival rates are short. Approximately 160,000 people worldwide are diagnosed with DLBCL each year.
Details of the STARGLO Study
The STARGLO study is a Phase III clinical trial that investigated the efficacy and safety of Columvi in combination with GemOx versus MabThera®/Rituxan® in combination with GemOx (R-GemOx) for people with R/R DLBCL who have received at least one prior line of therapy and are not candidates for autologous stem cell transplant, or who have received two or more prior lines of therapy.
The study’s primary analysis confirmed that the combination of Columvi and GemOx significantly improved overall survival rates (OS) compared to R-GemOx. Patients treated with Columvi plus GemOx lived significantly longer, with a 41% reduction in the risk of death compared to R-GemOx. Columvi also met its key secondary endpoints, with a 63% reduction in the risk of disease worsening or death (progression-free survival, PFS) compared to R-GemOx.
The Columvi combination demonstrated clinically significant improvement in overall survival rates, as well as key secondary endpoints, in patients with limited treatment options and who are ineligible for transplant. The combination’s benefits were reinforced with an additional 11 months of follow-up.
Columvi: A Promising New Treatment
The positive results from the STARGLO study represent a significant step forward in advancing Columvi combinations in earlier settings to address the urgent need for people who relapse or have refractory disease with limited treatment options. The treatment is particularly important for patients with highly aggressive disease who are at risk of rapid disease progression. Furthermore, patients do not have to wait to start treatment with Columvi, making it a viable treatment option for those who need it most.
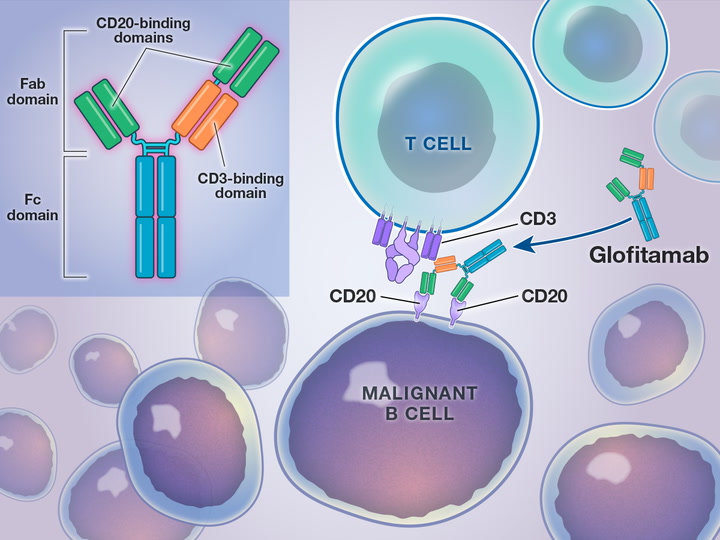
Columvi is the first CD20xCD3 bispecific antibody to demonstrate a survival benefit in DLBCL in a randomized Phase III trial. As a fixed-duration treatment, patients with R/R DLBCL have a treatment end date and the possibility of a treatment-free period – a stark contrast to continuous treatments.
Future of Columvi
The results from the STARGLO study will be submitted to global health authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency, to seek approval of the treatment. Columvi is also being investigated for other aggressive, hard-to-treat lymphomas and recently received FDA Breakthrough Therapy Designation for the treatment of adult patients with relapsed or refractory mantle cell lymphoma who have received at least two prior therapies based on results from the Phase I/II NP30179 study. STARGLO intends to confirm the accelerated approval in the US and conditional marketing authorization in the EU to full approvals for people with R/R DLBCL after two or more lines of systemic therapy based on the pivotal Phase I/II NP30179 study.
About Roche in Hematology
Roche has been developing medicines for people with malignant and non-malignant blood diseases for more than 25 years. Our experience and knowledge in this therapeutic area are extensive, and we offer innovative treatment options to patients across a wide range of hematologic diseases. Our approved medicines include MabThera®/Rituxan® (rituximab), Gazyva®/Gazyvaro® (obinutuzumab), Polivy® (polatuzumab vedotin), Venclexta®/Venclyxto® (venetoclax) in collaboration with AbbVie, Hemlibra® (emicizumab), Lunsumio® (mosunetuzumab) and Columvi® (glofitamab). Our pipeline of investigational hematology medicines includes T-cell engaging bispecific antibody cevostamab, targeting both FcRH5 and CD3, Tecentriq® (atezolizumab), and crovalimab, an anti-C5 antibody engineered to optimize complement inhibition.
Roche is the world’s largest biotechnology company and a global leader in in-vitro diagnostics. The company pursues scientific excellence to discover and develop medicines and diagnostics for improving and saving the lives of people around the world. Roche has been named one of the most sustainable companies in the pharmaceuticals industry by the Dow Jones Sustainability Indices for the fifteenth consecutive year.
Originally Post From https://www.stocktitan.net/news/RHHBY/roche-s-phase-iii-starglo-study-demonstrates-columvi-significantly-o3xlw2ubjizp.html
Read more about this topic at
FDA Approves Genentech’s Fixed-Duration Bispecific …
Bispecific antibodies for the treatment of B-cell lymphoma