Epcoritamab potentially provides promising results for patients with follicular lymphoma
Overview
Follicular lymphoma (FL) is the second most common form of non-Hodgkin’s lymphoma (NHL). Approximately 15,000 individuals develop FL each year in the United States, accounting for 20-30% of all NHL cases. FL is considered incurable with present standard of care therapies, and patients often relapse. Patients receiving currently available treatments may experience shorter durability of remission with every subsequent line of therapy. This emphasizes the need for innovative treatment options.
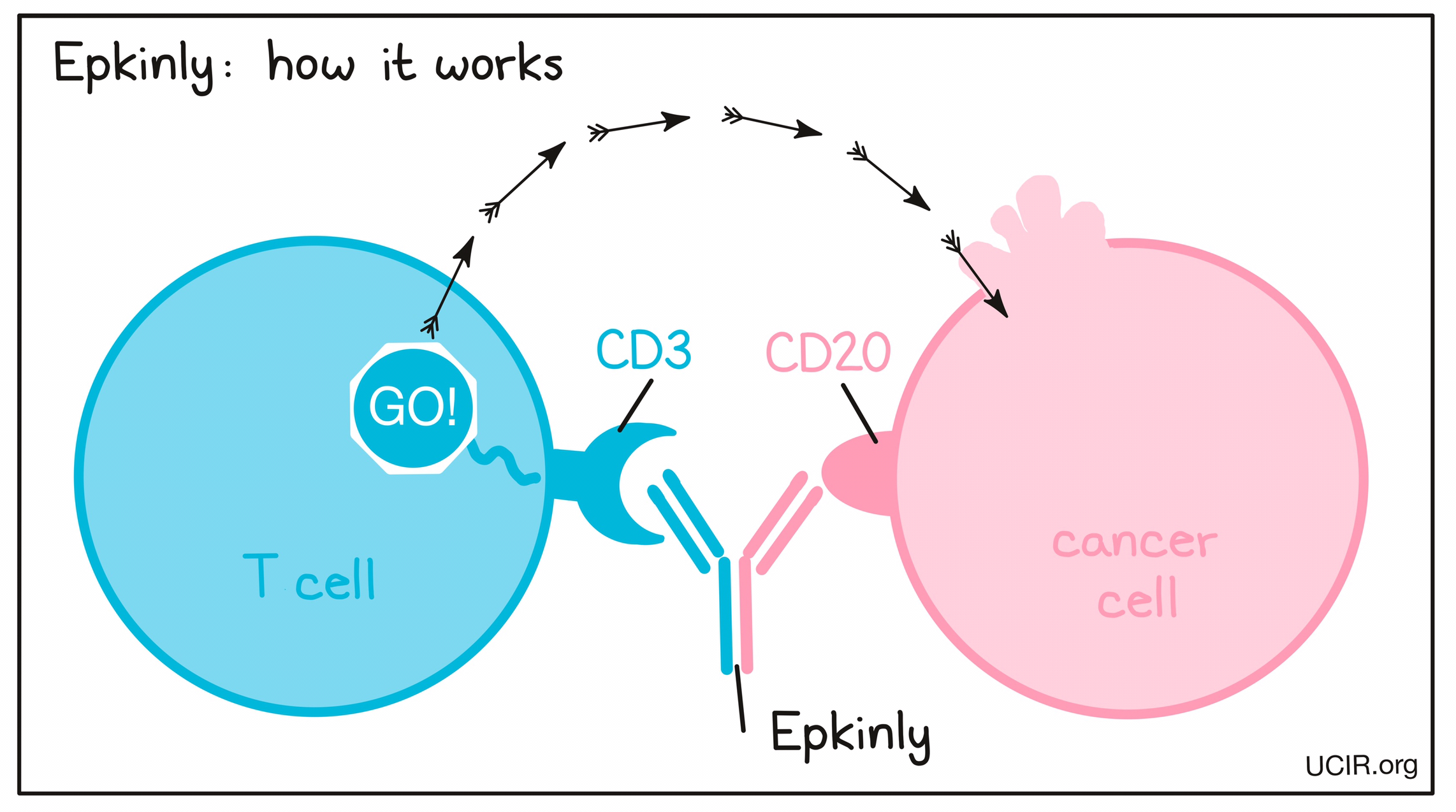
Epcoritamab, an IgG1-bispecific antibody created using Genmab’s proprietary DuoBody® technology, may potentially provide promising results for patients with FL, whether previously untreated or post-relapse.
Efficacy for Previously Untreated FL
The EPCORE NHL-2 study analysis included results from two arms. Arm 6 evaluated epcoritamab in combination with R2 (rituximab-lenalidomide) in patients with previously untreated FL (n=41). With a median follow-up of 21.1 months, additional findings from this arm showed durable responses. Specifically, an estimated 89% of patients remaining progression free, with 93% of patients who had achieved complete response (CR) remaining in CR at 18-months.
According to Joshua Brody, MD, Director of Lymphoma Immunotherapy Program at the Icahn School of Medicine at Mount Sinai Hess Center for Science and Medicine, “The results from this preliminary analysis of epcoritamab in combination with rituximab-lenalidomide as a first-line, chemotherapy-free treatment for patients with FL are encouraging and support the continued evaluation of epcoritamab in this patient population.”
Safety and Tolerability
In arm 6, the most common treatment-emergent adverse events (TEAEs) in first-line patients were COVID-19 (63%), cytokine release syndrome (CRS; 56%), and neutropenia (56%). All CRS events were low grade (41% Grade 1 and 15% Grade 2) and resolved. Most CRS events occurred after patients received their first full dose of epcoritamab, and none led to treatment discontinuation.
In arm 7, which evaluated epcoritamab administered every 8 weeks for patients with first- or second-line FL in CR or partial response (PR) following standard-of-care treatment (n=20), the most common TEAEs were COVID-19 (70%) and CRS (55%). Only one CRS event (Grade 1) occurred during Q8W maintenance dosing. One fatal treatment-emergent adverse event (TEAE) occurred related to respiratory failure caused by post-acute COVID syndrome.
In a C1 optimization cohort in patients with R/R FL, patients received epcoritamab in 3 step-up doses (0.16, 0.8, and 3 mg), along with dexamethasone prophylaxis and hydration recommendations, followed by full 48-mg doses until disease progression or unacceptable toxicity. With a median follow-up of 5.7 months, CRS rate was 49% (Grade 1 40%, Grade 2 9%), primarily occurring during cycle 1. There were no ICANS events.
Future Outlook
On February 26, 2024, the US Food and Drug Administration (FDA) granted Priority Review for the supplemental Biologics License Application (sBLA) for epcoritamab-bysp for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) after two or more lines of systemic therapy. Use of epcor
Read more about this topic at
EHA 2022: Investigational Epcoritamab Shows Promise in …
Epcoritamab Shows Promise in Large B-Cell Lymphoma
