Leads Biolabs’ Innovative Cancer Treatment LBL-024 Achieves Outstanding Phase II Results at 2024 ASCO Annual Meeting
The annual meeting of the American Society of Clinical Oncology (ASCO) commenced on May 31st, 2024, showcasing groundbreaking cancer research from around the world. Over 7,000 abstracts were submitted this year, with LBL-024, a bispecific antibody independently developed by Leads Biolabs, being selected for an oral presentation.
Promising Results for A Rare Cancer
LBL-024 has demonstrated good safety and strong efficacy signals in patients with advanced malignant tumors, particularly extrapulmonary neuroendocrine carcinomas (EP-NEC) patients who failed at least one line of chemotherapy. This is significant because EP-NEC is a rare and lethal malignancy, with no drug approved by regulatory agencies for this disease so far, and recommended treatment options being limited.
More About EP-NEC
EP-NEC typically occurs in the stomach, intestines, and pancreas, and patients are usually diagnosed at a later stage when the cancer has already metastasized to other areas in the body. As the disease progresses rapidly, the prognosis is extremely poor, and thus the urgency to develop novel therapeutic approaches is high.
LBL-024 Monotherapy Shows Promising Efficacy Data
During the Clinical Science Symposium-Building Novel Antibody-Based Approaches in Gastrointestinal Cancers, Dr. Panpan Zhang presented the outstanding clinical data of LBL-024 monotherapy. The phase I/II first in human, open-label, multicenter, dose escalation/expansion study evaluates the safety and efficacy of LBL-024 monotherapy in patients with advanced malignant tumors and neuroendocrine carcinoma.
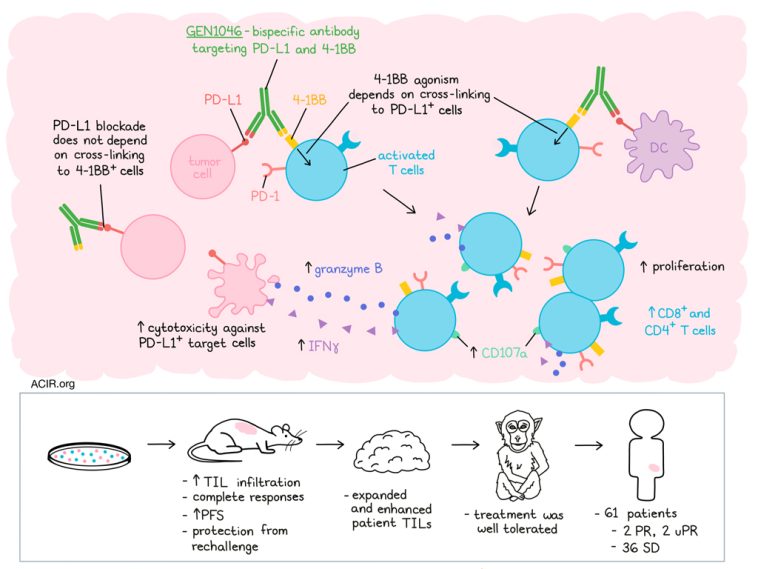
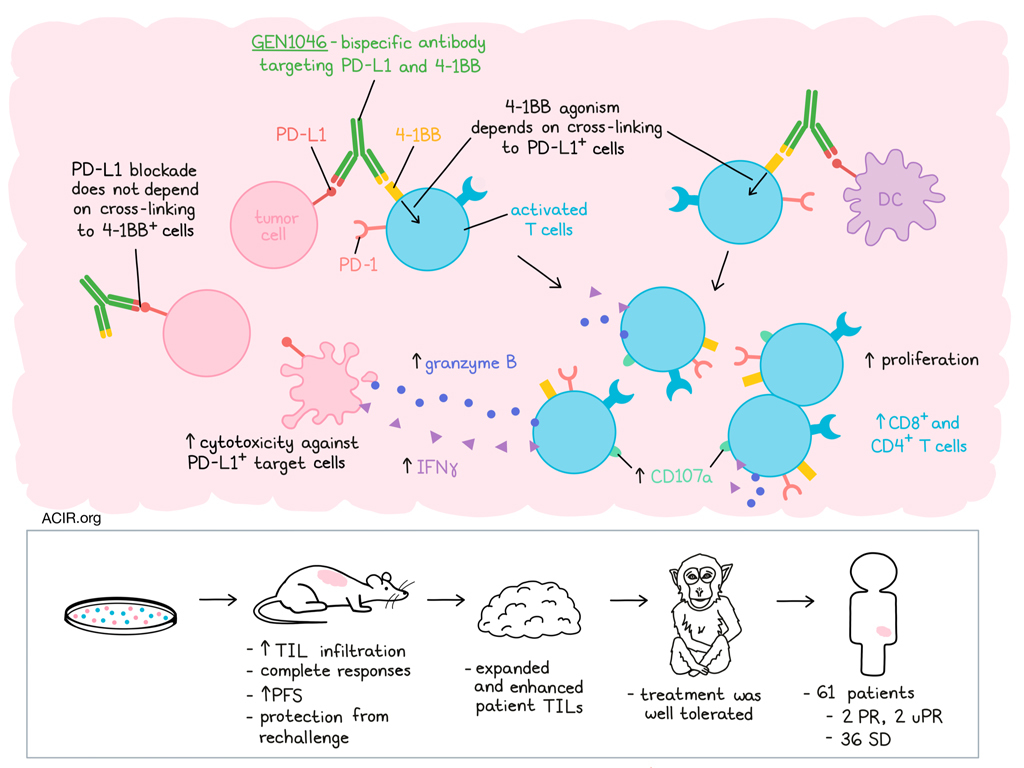
LBL-024 monotherapy demonstrated good safety profile, especially mild liver toxicity in advanced solid tumors. The severity of adverse events (AEs) was mostly grade 1-2, and no unexpected AEs were observed. At the dose of 15 mg/kg, the overall response rate (ORR) was 37.5% and the disease control rate (DCR) was 50.0% in 2nd line EP-NEC, and robust anti-tumor activities were observed in a wide therapeutic dose range (0.8-15mg/kg) in EP-NEC. The median follow-up was 8.5 months, the median duration of response (DoR) was 5.3 months, median overall survival (OS) was not reached, and the 6-months OS rate of the overall, 2nd line, and ≥3rd line were 61.7%, 72.7%, and 52.0%, respectively. A 54.5% ORR was observed in 22 patients with PD-L1 negative expression (CPS<1), indicating patients can benefit from treatment with LBL-024 regardless of PD-L1 expression in tumor tissue.
Continued Development of LBL-024
According to the study results, LBL-024 monotherapy showed good safety profile and very promising antitumor effects on advanced malignant tumors, particularly on EP-NEC, which support the continued development of LBL-024 in patients with EP-NEC. A single-arm pivotal study of LBL-024 monotherapy in EP-NEC was approved on April 30, 2024, which will help accelerate the listing process of LBL-024. Currently, there are no 4-1BB-targeting drugs available both domestically and internationally. LBL-024 has first-in-class potential and is expected to become the first approved standard treatment for EP-NEC after progression on second-line therapy, offering a more effective treatment option and providing hope for survival to patients with advanced EP-NEC.
About Leads Biolabs
Nanjing Leads Biolabs Co., Ltd. is a clinical-stage biotechnology company founded in Nanjing by a team of senior U.S.-trained antibody drug developers. Since 2014, Leads Biolabs has been dedicated to the discovery and development of novel antibody drugs with independent intellectual property rights for the treatment of oncology and other major diseases of high unmet medical needs, particularly the challenges in cancer immunotherapy. The extensive R&D pipeline of the company consists of more than twenty novel tumor immunotherapy, autoimmunity, and antibody-drug conjugates (ADCs) molecules based on monoclonal and bispecific antibody technology platforms.
LBL-024 is a uniquely designed bispecific antibody composed of an anti-Programmed Cell Death Ligand-1 (PD-L1) and an anti-4-1BB (CD137) antibody. It binds to PD-L1 with high affinity, blocks the immunosuppressive pathway of tumor cells by targeting PD-L1, and effectively localizes 4-1BB co-stimulation to the tumor microenvironment. This activation of T cells exerts a powerful immune response, resulting in a potentially stronger antitumor effect than anti-PD-1/PD-L1 monoclonal antibodies alone.
Conclusion
The development of novel therapeutic approaches for rare and lethal malignancies such as EP-NEC is crucial to provide hope for survival to patients. LBL-024 has demonstrated good safety and strong efficacy signals as a monotherapy in patients with advanced malignant tumors, particularly EP-NEC patients who failed at least one line of chemotherapy. With the approval of a single-arm pivotal study of LBL-024 monotherapy in EP-NEC and its expected potential as the first approved standard treatment for EP-NEC after progression on second-line therapy, LBL-024 offers a more effective treatment option to patients, providing hope for better outcomes in this challenging medical field.
Disclaimer: The content in this article is for informational purposes only and does not constitute medical advice. Please consult a licensed healthcare professional for diagnosis and treatment of any medical condition.
Read more about this topic at
Novel Cancer Treatment Shows Promise
Novel cancer treatment paradigm targeting hypoxia …