Developing Antibody-Drug Conjugates for Hard-to-Treat Cancers
Introduction
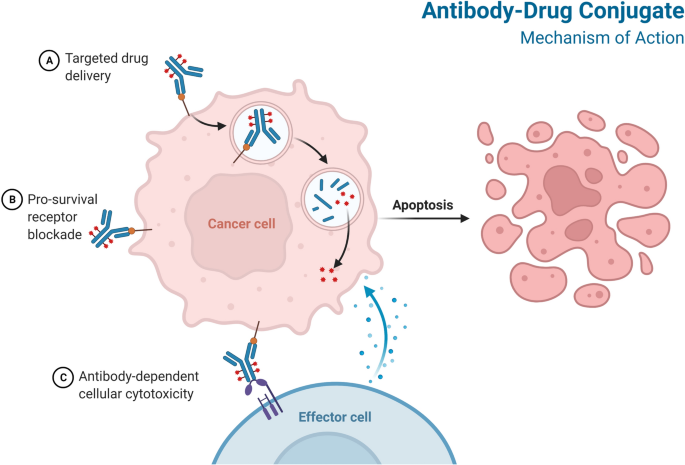
Antibody-drug conjugates (ADCs) have emerged as promising new therapies for the treatment of solid tumors and hematologic malignancies. Several FDA-approved ADCs have paved the way for new treatment options, while ongoing research continues to identify new targets and improve outcomes.
Promising ADCs for the Treatment of Cancer
Blenrep for Relapsed or Refractory Multiple Myeloma
Belanatamab mafodotin (Blenrep) is a BCMA-targeted ADC that received FDA accelerated approval in 2020 for the treatment of patients with relapsed or refractory multiple myeloma who have received at least 4 prior therapies. Data from the DREAMM-8 trial presented at the 2024 American Society for Clinical Oncology (ASCO) Annual Meeting showed promising median progression-free survival (PFS) results for the BPd arm compared to the PomDex plus bortezomib arm (P<.001). However, in January 2023, AstraZeneca announced plans to discontinue the agent due to poor clinical uptake, not due to any safety or efficacy issues.
Datopotamab Deruxtecan for HER2- Breast Cancer
Datopotamab deruxtecan (Dato-DXd; DS-1062a) is a therapy that has yet to receive FDA approval for patients with HER2- breast cancer. In a study of patients with HR+/HER2- metastatic breast cancer, treatment with Dato-DXd showed promising median progression-free survival results compared to physicians’ choice of chemotherapy. Dato-DXd has the potential to offer a longer PFS and a better safety profile compared with docetaxel.
Disitamab Vedotin for Locally Advanced or Metastatic Urothelial Cancer
Disitamab vedotin (formerly RC48) is an ADC composed of a HER2-targeted monoclonal antibody, herceptin, and monomethyl auristatin E (MMAE) via an mc-val-cit-PABC linker. The agent is also being studied in combination with the PD-1 inhibitor toripalimab (Loqtorzi), which demonstrated promising clinical activity with a manageable safety profile in patients with locally advanced or metastatic urothelial cancer.
AABB-011 for Small Cell Lung Cancer
AABB-011 is an ADC that was studied in a phase 1, open-label, dose-escalation study for the treatment of small cell lung cancer. The ORR was 10% for second line patients and 14% for third line patients, with the DCR at 65% and 73%, respectively. The most common treatment-emergent AEs were increases in aspartate levels and alanine aminotransferase, nausea, and fatigue.
Tusamitamab Ravtansine for Metastatic or Locally Advanced Solid Tumors
Tusamitamab ravtansine (SAR408701) is a promising ADC consisting of a humanized IgG1 antibody specific to CEACAM5 that has been conjugated to maytansinoid DM4. In a phase 1 trial, treatment with Tusamitamab ravtansine resulted in a disease control rate of 78.4% and a median duration of response of 7.6 months.
Conclusion
ADCs continue to be a promising area of research for the treatment of hard-to-treat cancers. Development of new agents, improved outcomes, and ongoing research are paving the way for new therapeutic options for patients.
Originally Post From https://www.targetedonc.com/view/adcs-on-the-horizon-offer-hope-for-challenging-cancers
Read more about this topic at
ADCs on the Horizon Offer Hope for Challenging Cancers
ASCO: Replacing chemotherapy with ADCs? AbbVie …