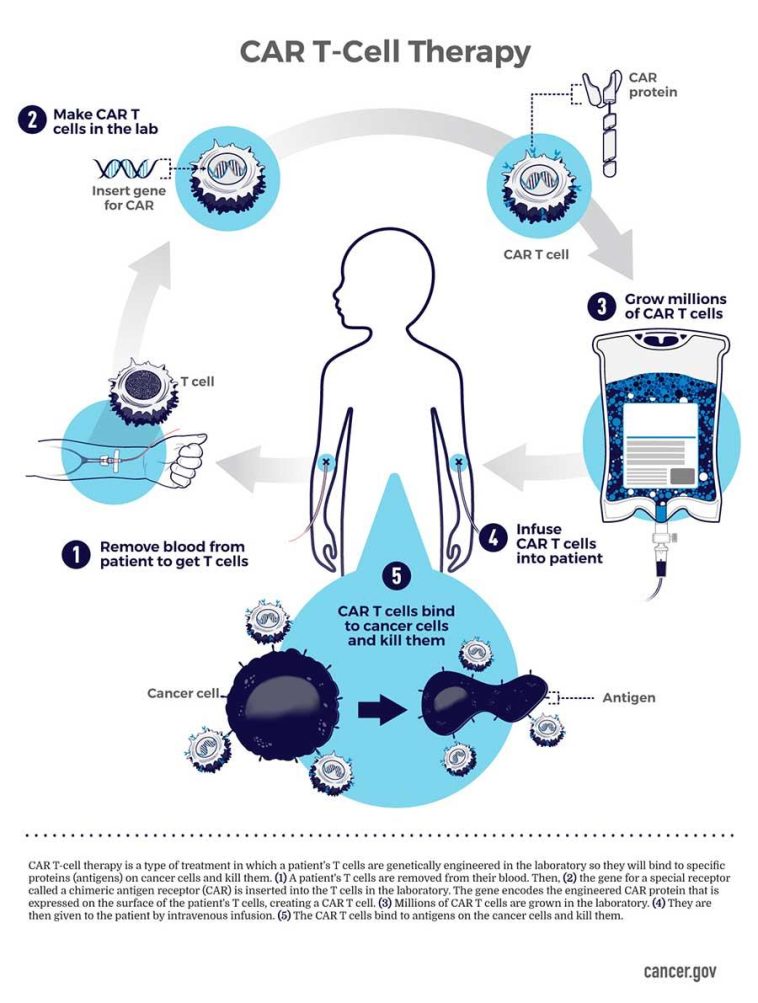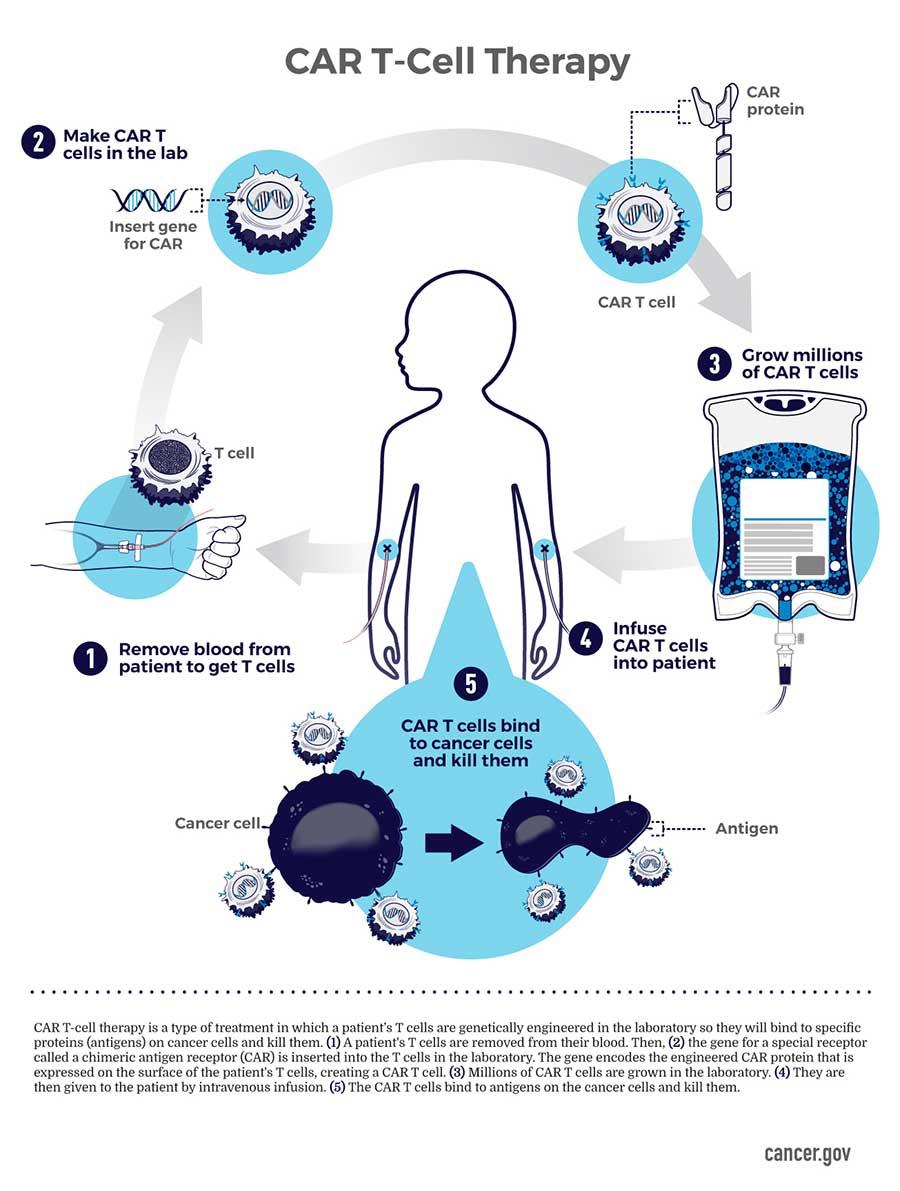
Novel CAR T cell therapy Obe-cel Yields Strong Remission Rates in Adults with Relapsed or Refractory B-ALL
Introduction
The Phase Ib/II FELIX clinical trial results demonstrated that the anti-CD19 autologous chimeric antigen receptor (CAR) T cell therapy obe-cel is an effective and safe treatment option for adults with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). The therapy achieved durable remissions in 40% of patients, who were in ongoing remission without the need for a subsequent stem cell transplant or other therapies.
Study Details
The study, funded by Autolus Therapeutics, involved treating 127 adult B-ALL patients with relapsed or refractory B-ALL with obe-cel. The patients underwent lymphodepletion followed by obe-cel infusion in split doses on days one and ten. The median follow-up time was 21.5 months.
Finding and Implications
The 12-month event-free survival (EFS) and overall survival (OS) rates were 49.5% and 61%, respectively. Persistence of CAR T cells and B-cell aplasia were associated with longer event-free survival in patients treated with obe-cel. The study also showed that SCTs did not offer additional value for these patients, suggesting that obe-cel could be considered a standalone treatment for adults with relapsed or refractory B-ALL. These findings demonstrate the potential for a long-term plateau in the survival curve and support obe-cel being considered a standard-of-care treatment for patients with limited treatment options.
Shorter Durations of Venetoclax Yields similar Response Rates in AML
Introduction
In a retrospective multi-center analysis, a 7-day course of venetoclax demonstrated similar remission rates as the standard 28-day course for older or chemotherapy-ineligible patients with newly diagnosed acute myeloid leukemia (AML). The shorter course was also found to be more tolerable with fewer side effects than the standard course.
Study Details
The analysis involved comparing data from 82 patients treated with the shorter 7-day venetoclax course to 166 patients treated with the current recommended 28-day course. The response rate was the same for both groups, with 72% of patients achieving composite complete remission.
Finding and Implications
The median overall survival for the shorter duration cohort was 11.2 months compared to 10.1 months for the longer duration cohort. The 8-week mortality rate was significantly higher in the 28-day treatment group compared to the 7-day group at 16% vs. 6%, respectively, suggesting that the shorter course was more tolerable, with fewer side-effects. The study supports the use of a shortened course of venetoclax in triplet regimens such as those being developed by MD Anderson researchers to treat older or chemotherapy-ineligible patients. This has the potential to reduce side-effects and improve patient tolerability without compromising response rates.
Conclusion
In conclusion, both studies show promising results in treating patients with relapsed or refractory B-ALL and AML, respectively, who have limited treatment options. The results of these studies add to the existing body of knowledge on the treatment of these conditions and present potential options for future treatments.
Originally Post From https://www.eurekalert.org/news-releases/1046615
Read more about this topic at
CAR T Cells: Engineering Immune Cells to Treat Cancer
CAR T-cell Therapy and Its Side Effects