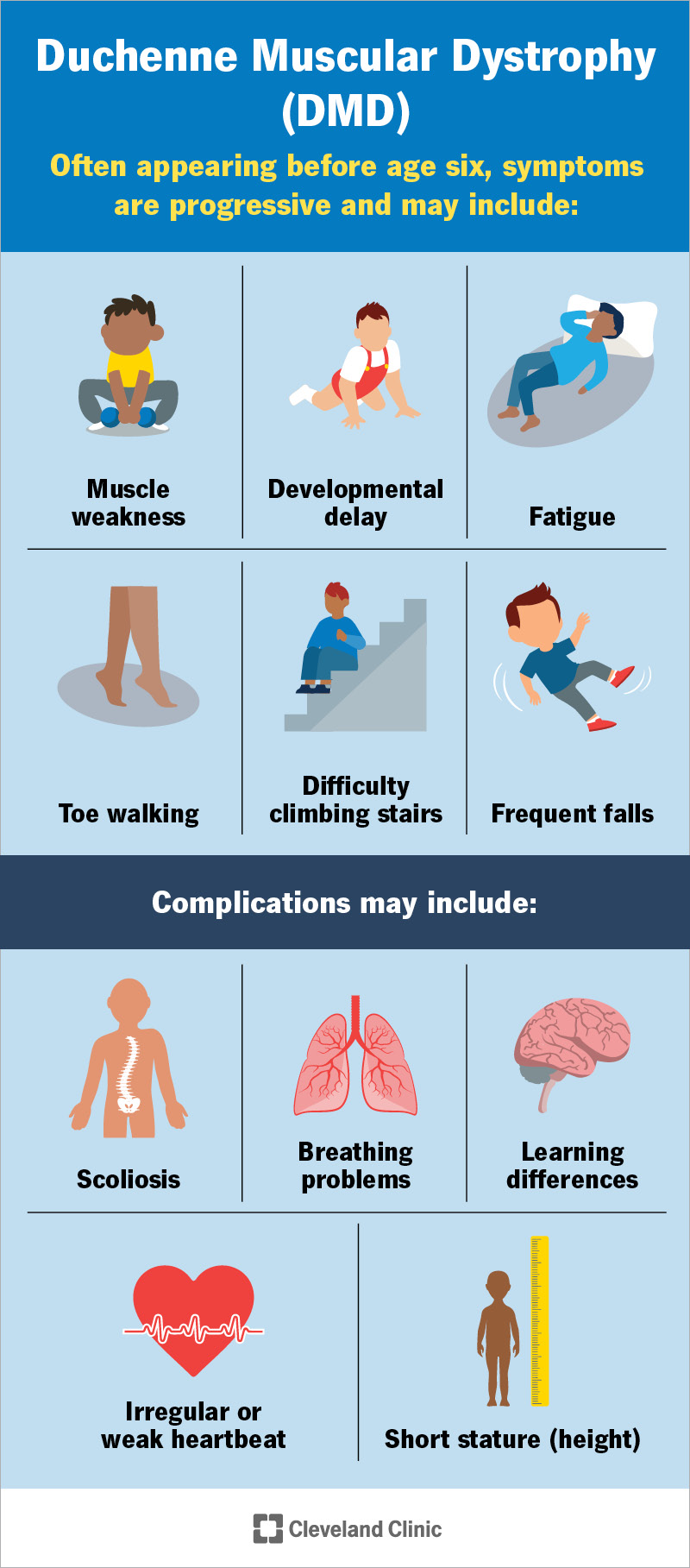
Sarepta Therapeutics Announces FDA Approval of ELEVIDYS
Sarepta Therapeutics, the leader in precision genetic medicine for rare diseases, has announced the expansion of the ELEVIDYS label to include individuals with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene who are at least 4 years of age. The FDA granted traditional approval for ambulatory patients, and the FDA granted accelerated approval for non-ambulatory patients. Continued approval for non-ambulatory Duchenne patients may be contingent upon verification of clinical benefit in a confirmatory trial.
The Impact of Gene Therapy on the Duchenne Community
“Representing many years of dedicated research, development, investment and creative energy, the expansion of the ELEVIDYS label to treat Duchenne patients aged 4 and above, regardless of ambulatory status, is a defining moment for the Duchenne community. Today also stands as a watershed occasion for the promise of gene therapy and a win for science,” said Doug Ingram, president and chief executive officer, Sarepta.
Jerry Mendell, M.D., co-inventor of ELEVIDYS and senior advisor, Medical Affairs, Sarepta, stated: “Today’s expansion of the ELEVIDYS label represents the culmination of my 50-year pursuit of a treatment for Duchenne patients and, along with my colleague Dr. Louise Rodino-Klapac, a nearly 20-year effort to optimize and develop a gene therapy that could be safely and effectively delivered to muscle.”
Impact of the Approval on the Treatment of DMD
ELEVIDYS is a single-dose, adeno-associated virus (AAV)-based gene transfer therapy for intravenous infusion designed to address the underlying genetic cause of Duchenne muscular dystrophy. ELEVIDYS is indicated for the treatment of Duchenne muscular dystrophy (DMD) in individuals at least 4 years of age. The DMD indication in non-ambulatory patients is approved under accelerated approval based on expression of ELEVIDYS micro-dystrophin in skeletal muscle.
This approval represents a significant milestone, and the expanded indication means clinicians now have a treatment option for the majority of boys and young men living with Duchenne. Moreover, this expansion speaks to the success of the science, the evidence, and the improvements in the trajectory of the disease across studies.
Collaboration and Ongoing Development
As part of a collaboration agreement signed in 2019, Sarepta is working with Roche to transform the future for the Duchenne community, enabling those living with the disease to maintain and protect their muscle function. Sarepta is responsible for regulatory approval and commercialization of ELEVIDYS in the U.S., as well as manufacturing. Roche is responsible for regulatory approvals and bringing ELEVIDYS to patients across the rest of the world.
Conference Call Details
At 8:30 a.m. ET on June 21, 2024, Sarepta will host a conference call and webcast to discuss this update. The event will be webcast live under the investor relations section of Sarepta’s website at https://investorrelations.sarepta.com/events-presentations. Interested parties participating by phone will need to register using the online form available on the website.
Risks Associated with ELEVIDYS
Sarepta Therapeutics cautions that risks include serious liver injury, immune-mediated myositis, and myocarditis. ELEVIDYS is contraindicated for patients with exon 8/9 deletions in the DMD gene.
Conclusion
This FDA approval is a significant milestone for the Duchenne community and a defining moment for gene therapy. The expanded indication means clinicians now have a treatment option for the majority of boys and young men living with Duchenne. As a leader in precision genetic medicine for rare diseases, Sarepta continues to spearhead the development of innovative treatments.
Originally Post From https://www.stocktitan.net/news/SRPT/sarepta-therapeutics-announces-expanded-us-fda-approval-of-elevidys-fqm8u28ib082.html
Read more about this topic at
Precision BioSciences: Home
A new age of precision gene therapy