CAR-T Therapy: A Revolutionary Cancer Treatment
The Trojan War and CAR-T Therapy: Similarities and Differences
CAR-T therapy is a personalised immunotherapy and cancer treatment that involves reprogramming the patient’s T-cells so they can recognise and attack cancer cells. Like the Trojan Horse, it infiltrates and overcomes the enemy, however, in the case of cancer, it does not destroy the healthy cells.
In 2006, a post-doctoral researcher delivered groundbreaking news to Prof Carl June at Penn Medical School in the USA, marking the beginning of an innovative approach to cancer treatment known as CAR-T therapy. The researchers had administered genetically engineered white blood cells called Lymphocytes transformed into killer cells targeting cancer cells, called CAR-T cells, into a mouse model half a year prior. Upon examination, the CAR-T had effectively killed the cancer cells.
This is the beginning of one of the most revolutionary approaches ever taken in cancer therapy and treatment- Immuno-Oncology.
The Birth Story
CAR-T therapy was used for the first time to treat a child who had Leukaemia in 2010.
Little Emily Whitehead was diagnosed with Acute Lymphoblastic Leukaemia (ALL) when she was just five years old. Despite conventional treatments, her condition worsened. Her parents opted for a clinical trial with CAR-T at the Children’s Hospital of Philadelphia in April 2012, which successfully cured her leukaemia.
What is CAR-T Therapy?
Traditionally, cancer treatments relied on surgery, chemotherapy, and radiation. Targeted therapies using molecular alterations in cancer cells have since emerged, alongside immunotherapy, which bolster the immune system to fight tumours, marking a significant shift in cancer treatment approaches.
CAR-T therapy is personalised for each patient, involving the collection of T cells from the patient, followed by laboratory re-engineering to express receptors targeting antigens like (CD19) on tumour cells. Laboratory-engineered T cells are expanded to millions and then infused back into the patient. If successful, these CAR-T cells continue to multiply in the patient’s body and recognise and kill the cancer cells through the engineered receptors attached.
Success of CAR-T Therapy
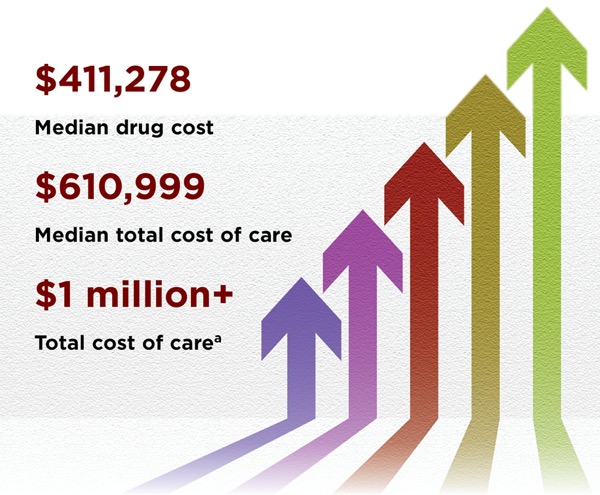
Since 2017, six CAR-T cell therapies have been approved by the FDA for treating blood cancers including lymphomas, some forms of leukaemia, and more recently multiple myeloma. However, the cost factor has been one of the biggest criticisms about CAR-T therapy, with the most recently approved one costing more than $450,000.
CAR-T Therapy in India
In response to the challenges faced by cancer patients with relapsing ALL who are unresponsive to chemotherapy and have limited treatment options, Prof Gaurav Narula, a paediatric oncologist at Tata Memorial Hospital, Mumbai, collaborated with Dr Rahul Purwar, a researcher from IIT Bombay. Since 2015, Rahul has been devoted to developing CAR-T therapy domestically in India.
After gaining insights from the National Institute of Health in Bethesda, Maryland, USA, the team successfully designed an effective CAR-T cell therapy named actalycabtagene autoleucel (NexCAR19). This therapy is customised to address the healthcare needs of India and can be locally manufactured at an estimated cost of $50,000. ImmunoACT, the spin-off company of IIT Bombay, will oversee manufacturing for the Indian market.
Initial short-term follow-up following the CAR-T infusions to patients with Lymphoma and Leukaemia (ALL) have shown promising results, says Dr Hasmukh Jain from TMH who presented the findings at the annual meeting in the USA. Recently, the president of India dedicated to the country the homegrown NexCAR19.
Decentralised Approach to CAR-T Cell Manufacturing
Under the guidance of Dr Vikram Mathews, Director of CMC Vellore, researchers have embarked on a decentralised approach to CAR-T cell manufacturing. They utilise a fully automated and closed Good Manufacturing Practice (GMP) compliant device called CliniMACS Prodigy, developed by Miltenyi Biotec, Germany.
Dr Vikram sees the advantage of producing CAR-T cells at the point of care using the CliniMACS Prodigy, ensuring adherence to required standards. Dr Sridhar Chaganti, programme director at the University Hospitals Birmingham NHS Trust’s CAR-T programme, views local CAR-T incubators as an option for large academic centres treating large numbers of patients but emphasises the requirement for a regulatory framework ensuring the quality testing of the product and assigning accountability to whoever is holding the license of manufacturing.
Dr Vikram envisions establishing a decentralised model across major tertiary care centres nationwide to meet patient needs. Additionally, the same automated CliniMACS Prodigy platform could potentially deliver gene therapies in the future for diseases like Thalassemia major and Sickle cell disease.
Risks Involved in CAR-T Therapy
CAR-T treatment may fail for several reasons. Dr Sattva Neelapu, a professor at the University of Texas MD Anderson Cancer Centre, Houston, USA, who led a key trial resulting in the first CAR-T approval for lymphoma in the USA, explains that it can occur if the targeted antigen in the tumour is reduced or if the T cells become less functional due to the chemotherapies that patients receive or due to the immune suppressive effect of the tumour on T cells.
Additionally, the treatment is linked to complications like Cytokine Release Syndrome (CRS) or a surge of inflammation and neurological toxicity. The treatment has an overall mortality rate of 10-12%. Prolonged immune suppression from the treatment can lead to infections such as pneumonia and viral infections.
Dr Sridhar advises informing patients about these risks and the potential need for protein concentrate (Immunoglobulin) administration to mitigate infection risks that can increase the cost of overall care.
CAR-T therapy is still at the initial stages in healthcare but it is undergoing exponential growth. It is hoped that it will transform cancer treatment and cancer care for patients globally.
Originally Post From https://www.deccanherald.com/health/towards-an-affordable-car-t-therapy-3056952
Read more about this topic at
CAR-T cell combination therapy: the next revolution in …
CAR T-Cell Therapy: Revolution in Cancer Therapy